The Studies
The Hearing Research Group is one of the leading Auditory Neuroscience groups in the United States, with ten laboratories studying auditory processing.
Our group seeks to deepen the understanding of how the ear and the brain function in association with hearing and communication across the lifespan, how they are affected by hearing disorders, and how they may be manipulated to prevent or treat these disorders.
NEOMED researchers study several hearing & communication health issues
- Age-related hearing loss
- Noise-induced hearing loss
- Hearing loss during development
- Auditory processing disorder
- Tinnitus
- Emotional disorders in speech communication
- Organization of the normal hearing brain
A patient’s perspective on hearing research
Katelyn shares her story of living with hearing loss and the importance of research on causes, prevention and treatment of hearing disorders.
VIDEO EXTRA: The Hearing Research Focus Area at NEOMED seeks to deepen the understanding of how the ear and the brain function in association with hearing and communication across the lifespan, how they are affected by hearing disorders, and how they may be manipulated to prevent or treat these disorders.
The Statistics
15%
Of American adults (37.5 million) report some trouble with hearing.
1 in 12
Nearly 1 in 12 U.S. children ages 3-17 have had a disorder related to voice, speech, language or swallowing in the past 12 months.
50M+
Over 50 million Americans experience some form of tinnitus (ringing in the ears).
The Successes
Lu lab
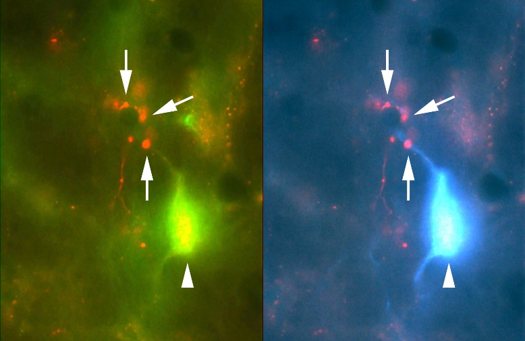
Cellular and molecular mechanisms underlying neuromodulation by metabotropic glutamate receptors in sound localization circuits.
Mellott Lab
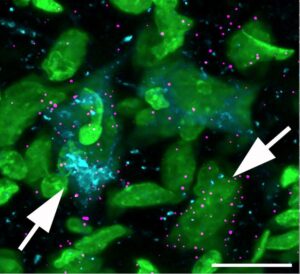
We discovered that auditory midbrain cells (arrows) heavily upregulate specific receptors (magenta) during middle-age and the subsequently downregulate the receptor during old age. The loss of these receptors in old age may underlie aspects of age-related hearing loss
Rosen lab
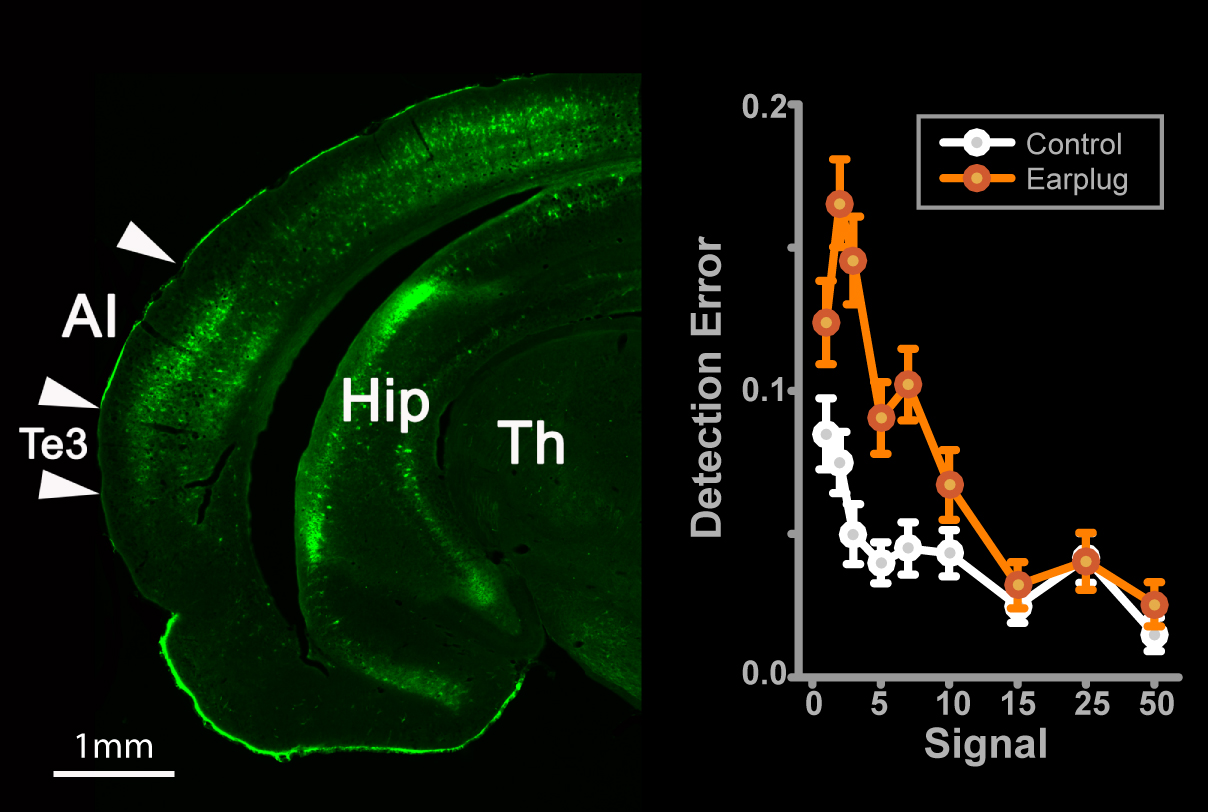
Early childhood hearing loss can result in long-lasting problems with speech perception. We discovered a novel method of remediating perceptual problems that also improved neural sensitivity in the auditory cortex.
Wenstrup Lab
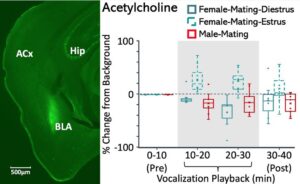
In response to emotional vocalizations, the neuromodulators acetylcholine and dopamine are released into the basolateral amygdala with context-dependent and estrous-stage dependent patterns. These patterns, revealed in studies using microdialysis combined with LC/MS chemical detection, likely provide the basis for context-dependent processing of social vocalizations by the amygdala.
Huyck Lab

This research is focused on the mechanisms underlying maturational changes in performance on basic auditory and speech perception tasks during adolescence in normal-hearing listeners. It will yield experimental protocols that will be applicable for the development of diagnostic tests regarding auditory processing in adolescents and young adults and provide insights for the development of rehabilitation strategies to treat disorders affecting auditory processing in adolescents and young adults.

The Scientists
Neural mechanisms underlying tinnitus, and potential therapies
Mechanisms of neurotransmission underlying auditory processing
Auditory perception and learning during adolescence
Nichole Beebe, Ph.D.
Research Assistant and Professor of Anatomy and Neurobiology
Age-related circuit changes that occur before hearing loss

Age-related factors that affect speech perception
Effects of stress and hearing loss on auditory perception and neural circuits
Neuroanatomical and neurochemical analysis of auditory circuitry
Neural circuits underlying emotional vocal communication
Cellular properties of neurons that support sound localization
The Stories
- Katelyn was born about four weeks prematurely. Though she passed a newborn hearing screen, she would later be diagnosed with enlarged vestibular aqueduct and mild cochlear dysplasia. EVA is a [...]Read the Story
- Read the Story
Mitch O’Hara, a research assistant who works in the lab of Jeffrey Mellott, Ph.D., was recently named the Seven Hills Hall of Fame Resident of the Month for August, 2021. […]
- Read the Story
When George Crumb composed Vox Balaenae (Voice of the Whale), he called for the small group of performers to wear black masks as they played, to symbolize “the powerful impersonal forces […]
Contact
Merri Rosen, Ph.D.
Director, Hearing Research Group
Phone: 330.325.6516
Email: mrosen@neomed.edu