Research
The Department of Pharmaceutical Sciences focuses on interdisciplinary investigation of pathophysiological mechanisms of chronic illnesses, identification of novel therapeutic targets, drug discovery and development. Faculty research areas include Alcoholic liver disease, Cancer, Metabolic disorders, and Neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, Traumatic brain injury, and Vision-related neurodegeneration.
The Department of Pharmaceutical Sciences is driven by its commitment to pursue educational excellence and advanced research, and ultimately aims to address the current healthcare issues facing society, while enhancing the knowledge of our students/scholars through classroom/laboratory experiences, discoveries and innovations.
The Neurodegenerative Disease and Aging Research Focus Area is housed within the department. Faculty conduct research in collaboration with colleagues in the College of Medicine, College of Graduate Studies, NEOMED-partner Universities, as well as investigators from national and international institutions.
Please contact department faculty for details on research, collaboration, and mentorship.
Christine Crish Lab
What can early changes to bodily homeostasis tell us about the beginning of the Alzheimer’s disease process in the brain?
Chemical signaling pathways that promote cell function in bodily tissues as well as in brain are disrupted early in AD, and symptoms like bone loss may be among the earliest indicators of these problems. We are determining whether detecting these early changes and restoring this signaling could delay or prevent onset of Alzheimer’s.
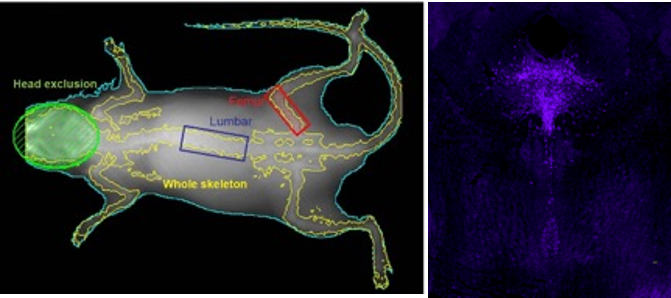
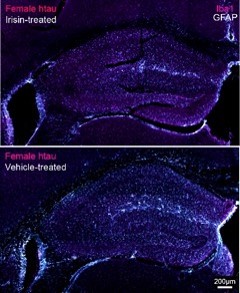
What makes exercise beneficial in reducing AD risk?
We are testing whether the newly discovered exercise-induced hormone irisin protects the brain from AD pathology and decreases the inflammatory impact of midlife metabolic risk factors for AD.
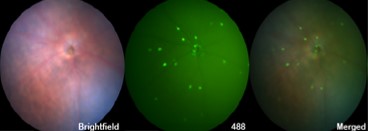
Are the eyes a window into the Alzheimer’s brain?
Amyloid beta pathology appears in retina before it does in brain, but little is known about how this impairs vision in patients. We are testing how this pathology affects vision and using the unique environment of the visual system to test drugs that may slow Alzheimer’s pathology.
Altaf Darvesh – Research Interests
Oxidative stress and inflammation are implicated in the pathophysiology of neurodegenerative diseases, as well as psychiatric illnesses. Our research focuses on the development of therapeutic strategies for the aforementioned ailments utilizing antioxidants, and anti-inflammatory agents.
- The development of therapeutic strategies for neurodegenerative diseases and psychiatric illnesses utilizing antioxidants, and anti-inflammatory agents.
- The investigation of the neuroprotective potential of natural products such as dietary polyphenols, e.g. curcumin, resveratrol, and tea catechins.
- The elucidation of neurochemical mechanisms involved in neurotoxicity of amphetamines.
- The development, implementation, and evaluation of the effectiveness of pharmaceutical sciences courses.
Sheila Fleming Lab
The Fleming lab conducts basic and translational research on Parkinson’s disease (PD), the common neurodegenerative movement disorder. Parkinson’s disease is part of a group of neurodegenerative disorders characterized by the abnormal accumulation of the presynaptic protein alpha-synuclein in lewy bodies and lewy neurites called synucleinopathies. We make use of tools and methods supplied by genetics, molecular biology, and behavioral neuroscience, all in an effort to identify mechanisms underlying abnormal protein accumulation and neurodegeneration in synucleinopathies. In the laboratory we test potential neuroprotective therapies using multiple models of PD. Here, we employ a battery of motor and non-motor behavioral assays to determine therapeutic efficacy and in the brain we measure PD-related pathology including alpha-synuclein aggregation, autophagy, inflammation, and mitochondrial efficiency. In addition to preclinical research, we also study gene-gene and gene-environment interactions related to alpha-synuclein pathology.
Ryo Fujiwara lab
Dr. Fujiwara’s lab investigates the metabolic and toxic reaction of new generation synthetic cannabinoids. They utilize various in vitro tools and equipment to identify the structure of metabolites and metabolic reactions such as recombinant enzymes, liver microsomes, HPLC, UPLC, and mass spectrometry. Recently, Dr. Fujiwara and his team members characterized the metabolic process of a third generation SCB, FDU-PB-22. After the phase I metabolism, the intermediate metabolite is conjugated into a hydrophilic metabolite by a phase II enzyme UDP-glucuronosyltransferase 1A10.
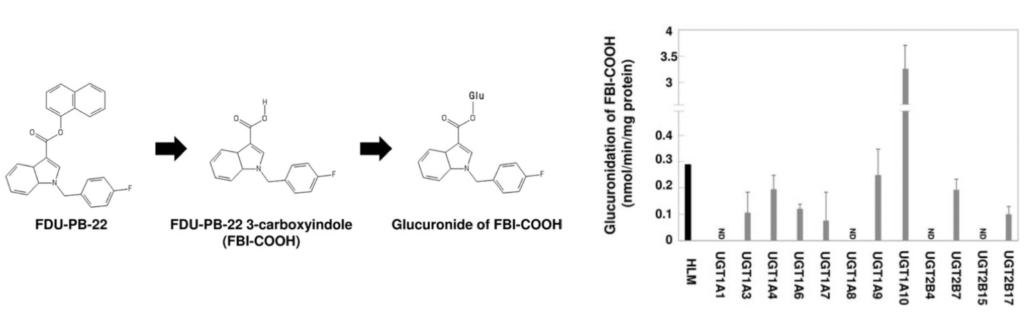
Takhar Kasumov Lab
Kasumov’s lab is taking a comprehensive/ collaborative approach to understand metabolic diseases.
Stable isotope tracers may yield novel insight regarding human biology. Methods developed in Kasumov’s lab allow translational studies, including studies in free-living subjects.
In collaborations with colleagues from Cleveland Clinic, Kasumov’s lab examines the role of altered protein homeostasis in fatty liver diseases and diabetes pathogenesis.
Moses Oyewumi Lab
The overarching goal of our work is to design, engineer and apply novel delivery systems as effective strategies to overcome barriers confronting therapeutic efficacy and application of drugs in the treatment/management of different diseases.
Erin Reed-Geaghan Lab
The overarching theme of the Reed-Geaghan lab is to elucidate the inflammatory changes that occur in Alzheimer’s disease. We use a variety of cellular and molecular approaches to study microglia, the resident immune cell of the brain, and their interactions with other cell populations for the initiation and propagation of Alzheimer’s disease pathogenesis. The long-term goal is to reveal avenues for new effective therapeutics to combat this devastating disease.
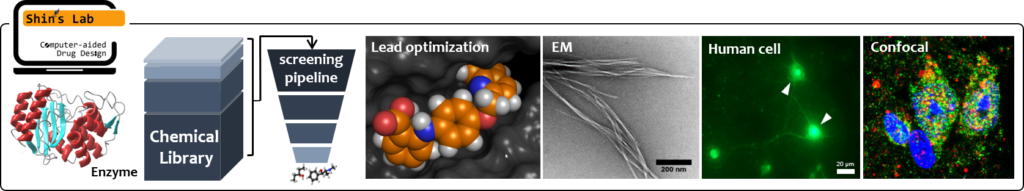
Woo Shik Shin Lab
Novel combination antibacterial therapy against drug resistance ESKAPE pathogens.
The major goal of the project is to develop a novel class of potent β-lactamase inhibitors to rescue existing β-lactam antibiotic activities for combination antibacterial therapy. The ability to preserve the efficacy of existing β-lactam antibiotics arsenal provides maximum opportunity for combination antimicrobial therapy development. To achieve this objective, we will use a state-of-the-art drug design methodology involving computational molecular modeling, cell & enzyme-based study, and chemical synthesis to generate inhibitors of a β-lactamase protein.
Drug repurposing and combinational approach to block abnormal protein aggregation in Alzheimer’s disease
Our lab focuses on computational structure-based drug design and drug delivery system for Alzheimer’s, Parkinson’s disease and other degenerative disorders. Current research is driven by two key topics: How to develop new therapeutic approach for neurodegenerative and other brain diseases? How to design the blood-brain barrier shuttle peptides with neuronal specificity?
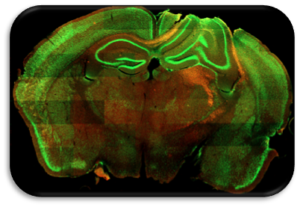
Matt Smith Lab
Research focus
To understand the neurobiological mechanisms that disrupt eye-brain communication in injury and disease. Using blend of classic neuroanatomical and neurophysiological techniques and new/innovative high-resolution correlative microscopy approaches, my lab can identify and assess structural and functional changes occurring to injured/diseased neurons.
Current Projects
We are exploring the role of a subset of retinal neurons, ipRGCs, in adolescent traumatic brain injury (TBI). We posit that pre-degenerative alternations to these specific, unique, and vulnerable neurons are likely responsible in part for the visual and vision-influenced symptoms commonly experienced by patients after brain trauma.
Long-term Objective
To improve diagnostic capabilities through developing approaches that take advantage of the relative accessibility of the eye to isolate and track neuron dysfunction occurring in the brain.
Xinwen Wang Lab
One size does not fit all. For many patients, standard drug treatment might not work or even trigger life-threatening adverse reactions. There is an urgent clinical need to provide tailored therapy for patients.
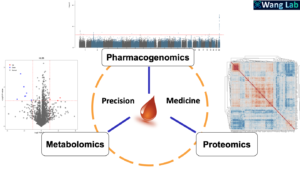
Our laboratory focuses on applying pharmaco-omics tools (i.e. pharmacogenomics, proteomics and metabolomics) to identify both genetic and non-genetic factors contributing to the interindividual variability related to disease risks and drug therapies, and to further pursue the underlying molecular basis for the interindividual variability in disease susceptibility and drug response, in order to eventually optimize the treatments for patients through genotype, prototype and metabotype guided precision medicine.
Our research is expected to improve the efficacy and safety of drugs by identifying biomarkers that can predict individual drug responses, revealing novel drug targets and translating these findings into clinical precision pharmacotherapy.
Contact
Cheri Harris
Business Manager
Phone: 330.325.6689
Email: charris1@neomed.edu
Department Chair
Moses Oyewumi, Ph.D.
Phone: 330.325.6669
Email: moyewumi@neomed.edu


